2025 Year End Report
Montreal, Quebec – December 23, 2025
2025 was a year defined not by stillness, but by motion. In a challenging biotech environment, Thryv Therapeutics continued to move forward – advancing our science, strengthening our organization, and staying focused on what matters most: delivering meaningful innovation for patients.
Over the past year, we strengthened our leadership team, deepened our engagement with the patient and advocacy communities, and continued to build the clinical, operational, and scientific foundation required to support Phase 2/3 development. Importantly, we also expanded how we listen to patients – recognizing that meaningful innovation depends on understanding not only clinical endpoints, but on lived experience.
Entering 2026, we are moving forward with clarity and focus, guided by the same commitment that carried us through the year.
We are deeply grateful to our employees, advisors, partners, investors, and the patients and families who inspire our work every day. Thank you for continuing this journey with us.
2025 Highlights
Operationalized our Phase 2/3 registration study for THRV-1268 for people with Long QT Syndrome enabling readiness to begin enrollment in the new year.
Completed our phase 1B study for THRV-1268 in obese participants that demonstrated predictable PK and reductions in QT interval confirming target engagement; supporting potential clinical benefit in heart failure and LQTS.
Launched myQTwave, a first-of-its-kind, fully remote, non-interventional study in Long QT Syndrome, enrolling up to 50 participants in our first wave of recruitment.
Strengthened leadership and organizational capabilities to support growth.
Continued collaboration with patient advocacy organizations, including the SADS Foundation.
Shared scientific progress at leading cardiovascular conferences.
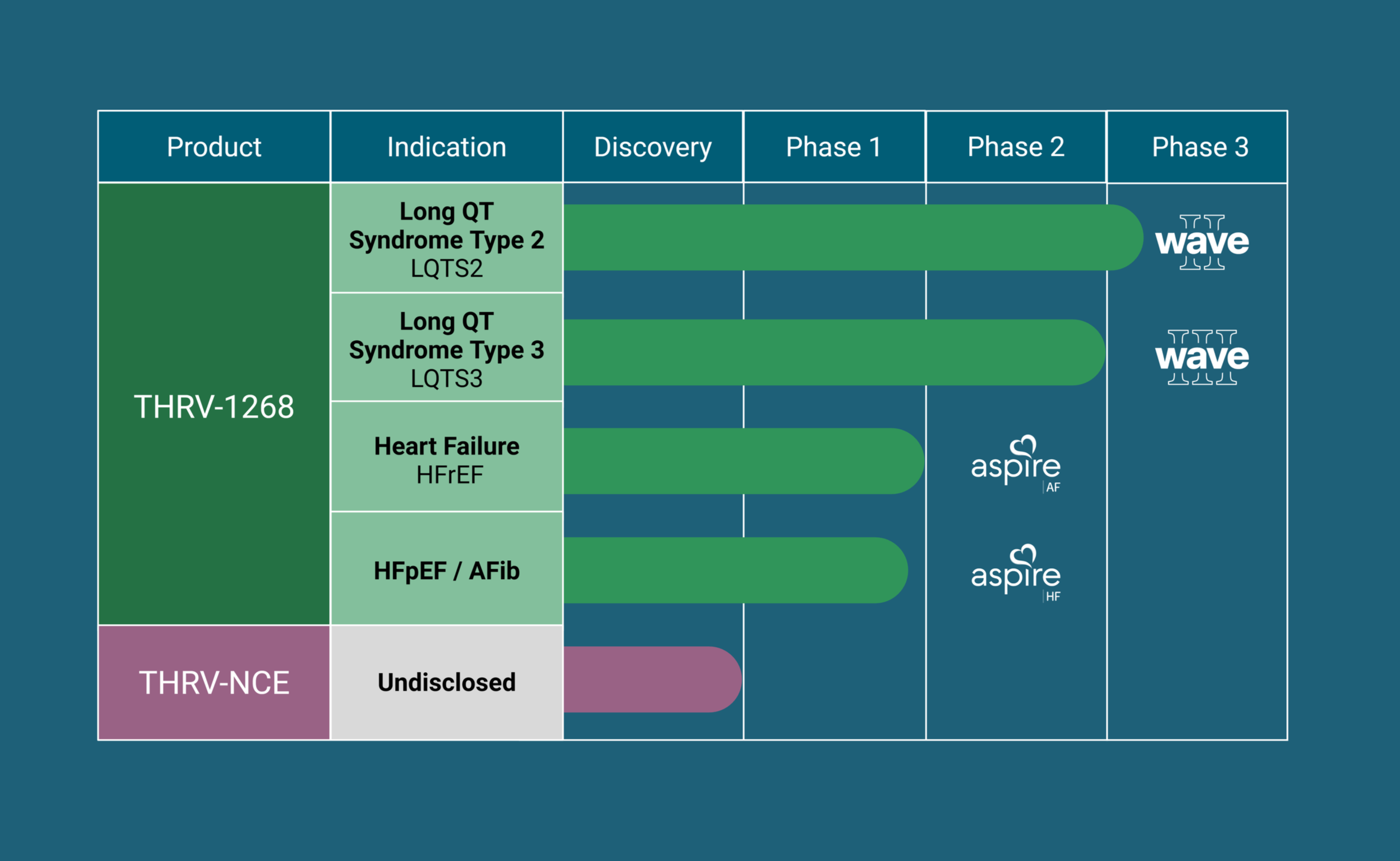
Pipeline Progress: THRV-1268
THRV-1268 is a novel, highly selective inhibitor of serum- and glucocorticoid-regulated kinase 1 (SGK1) being developed for the treatment of inherited cardiac arrhythmias, including Long QT Syndrome (LQTS), with broader applicability across cardiometabolic and cardiomyopathic disorders. By targeting SGK1, a central regulator of cardiac ion channel activity, cellular stress responses, and maladaptive remodeling, THRV-1268 is designed to address the underlying electrophysiologic and structural drivers of disease, rather than symptom control alone.
In 2025, Thryv advanced the program by building on robust Phase 1 clinical experience, integrating safety, pharmacokinetic, and pharmacodynamic data to inform Phase 2/3 trial design, dose selection, and patient-focused efficacy endpoints. These efforts enabled refinement of exposure–response relationships and reinforced confidence in target engagement in people with metabolic stress - obesity. Our development strategy remains grounded in a strong mechanistic and translational framework, linking SGK1 inhibition to clinically meaningful improvements in repolarization biology and disease severity, while maintaining a favorable safety profile.
Preparing for Phase 2/3 Development
Throughout the year, Thryv focused on activities critical to later-stage development, including obtaining regulatory clarity, protocol refinement, and opening two INDs (for LQTS and HF). We value continued input from a widened group of clinical and scientific advisors. These efforts position the company for efficient and rigorous execution in upcoming studies.
Elevating the Patient Voice: myQTwave
In 2025, Thryv advanced myQTwave from concept into execution, beginning with a pilot study and expanding enrollment to include more individuals living with Long QT Syndrome Types 2 and 3.
Conducted in collaboration with Dr. Samuel F. Sears Jr. and his team at East Carolina University (ECU), myQTwave is a fully remote, observational study for adults with LQT2 and LQT3. Using digital health tools such as iPhone and Apple Watch, the study captures patient-centered insights beyond traditional clinical metrics – such as ECG findings and cardiac events – to better understand how LQTS shapes daily decisions, quality of life, and emotional well-being. Insights from myQTwave will help inform future research and development.
Advocacy & Community Engagement
Patient and clinician engagement remained central to Thryv’s work in 2025. We deepened our collaboration with the SADS Foundation through patient-focused and electrophysiology (EP)-focused educational webinars, supporting awareness and dialogue around inherited arrhythmias.
Leadership & Team Growth
In 2025, Thryv continued to strengthen its leadership and operational teams to support the next phase of clinical and corporate development.
This included the appointment of Matt Killeen, PhD, as Chief Business Officer, bringing deep experience across cardiovascular and genetic diseases, business development, and portfolio strategy. Matt plays a key role in shaping Thryv’s corporate strategy and advancing THRV-1268 toward later-stage development.
We also welcomed Dinesh Srinivasan (Vice President, Strategic Portfolio Management), Ashley Prasse Miller (Vice President, Clinical Operations), Alicia Schiavi (Associate Director, Clinical Operations), and Philippe Beauclair (Postdoctoral Researcher, Chemistry), further strengthening our capabilities across clinical development, operations, and portfolio execution.
Scientific & Industry Engagement
In 2025, Thryv shared scientific progress at leading cardiovascular conferences, highlighting the growing body of evidence supporting SGK1 inhibition across cardiac disease.
At the American Heart Association (AHA) Scientific Sessions and European Society of Cardiology (ESC) Congress, Thryv presented preclinical data demonstrating that THRV-1268, a potent and selective SGK1 inhibitor, improved adverse cardiac remodeling in a transaortic constriction (TAC) heart failure model. These findings showed superior or complementary effects compared with Jardiance (empagliflozin) – an SGLT2 inhibitor used as part of guideline-directed medical therapy – both as monotherapy and in combination, strengthening the scientific rationale for SGK1 inhibition as a strategy to address inflammation- and fibrosis-driven cardiac remodeling.
At Heart Rhythm Society (HRS) 2025, Thryv presented new clinical data from the Wave I, Part 2 study demonstrating the effects of QT shortening with SGK1 inhibition treatment in patients with Long QT Syndrome Types 2 and 3.
Looking Ahead
The launch of myQTwave comes as Thryv advances multiple IND programs. The company has recently opened multiple INDs and is preparing to launch Wave II, a Phase 2/3, registration-directed clinical study in patients with Long QT Syndrome, anticipated to begin in early 2026.
In parallel, Thryv continues to expand the translational and clinical foundation of its SGK1 platform. Recent Phase 1 results in obese individuals further strengthened confidence in the program and informed ongoing development plans. Thryv is also advancing the Aspire Study program, a clinical initiative designed to further evaluate the role of SGK1 inhibition across cardiometabolic disease, including heart failure.
Together, these efforts underscore the continued advancement of THRV-1268 across multiple indications, as illustrated in the pipeline below.
Wishing all our supporters, patients, and families a healthy and happy new year!
Sincerely, Debra Odink
President & Chief Development Officer