Advancing Precision Medicine in Cardiology
At Thryv Therapeutics, we are leading a precision medicine approach to treat Long QT Syndrome (LQTS), heart failure, and atrial fibrillation. Our work is centered on developing novel, potent, and highly selective inhibitors of Serum Glucocorticoid-Inducible Kinase 1 (SGK1). By targeting SGK1, we aim to deliver innovative, disease-modifying therapies for patients with limited or no targeted treatment options.
Addressing the Unmet Medical Need in Long QT Syndrome (LQTS)
Long QT Syndrome (LQTS) is a life-threatening genetic arrhythmia condition that increases the risk of sudden cardiac events such as syncope, seizures, and even sudden death. Current treatment strategies — including beta-blockers, implantable devices, and lifestyle changes — provide partial protection and are not suitable for all patients. There are currently no regulatory-approved therapies specifically for LQTS. Our investigative SGK1 inhibitors are being developed to meet this urgent, unmet need with a novel, precision-based therapeutic approach.
myQTwave: Elevating the Patient Voice
As part of our commitment to advancing care for people living with Long QT Syndrome, we have launched myQTwave, a non-interventional, observational effort designed to better understand the lived experience of those affected by LQTS Types 2 and 3. By capturing patient-reported outcomes and aspects of daily life with the condition, this initiative will provide vital insights to guide our development programs and ensure that the patient voice remains central to our work. Enrollment is now open, and we look forward to partnering with the LQTS community to advance this important research.
Our Progress
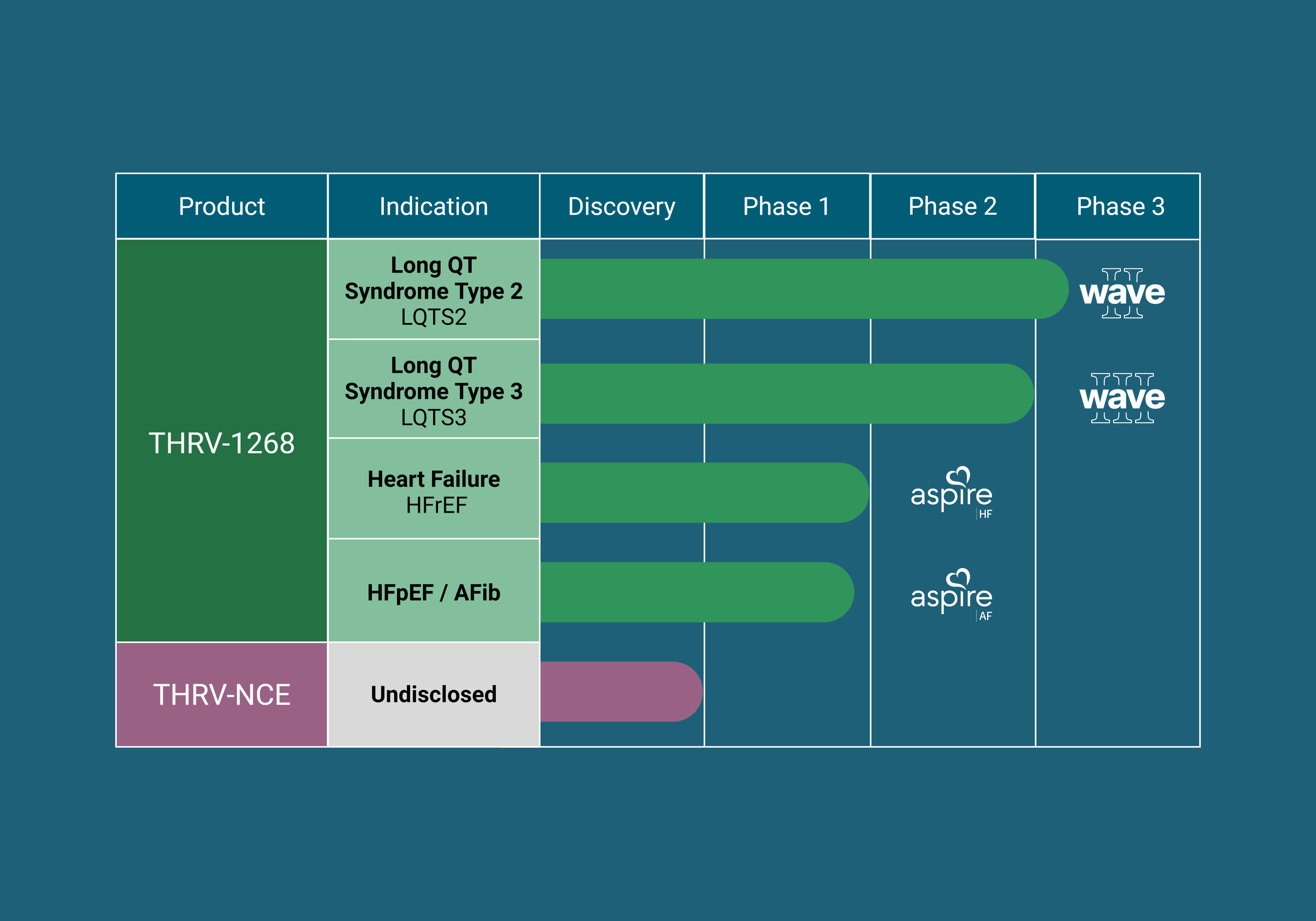
Our lead investigational SGK1 inhibitors are progressing through clinical development, guided by scientific innovation, patient need, and the principles of precision medicine. Parallel research efforts continue in our laboratories to identify next-generation SGK1 inhibitors that address additional cardiac conditions.
Clinical Highlights:
Preclinical studies completed
Phase 1 clinical study completed
Wave Clinical Studies:
Wave I, Part 1: Our investigational SGK1 inhibitor, LQT-1213, demonstrated rapid reductions in QTcF in healthy volunteers with dofetilide-induced QT prolongation.
Wave I, Part 2: LQT-1213 demonstrated statistically significant and clinically meaningful reductions in QTcF in patients with congenital Long QT Syndrome Types 2 and 3, supporting the initiation of Wave II in 2026.
Wave II: Pivotal Phase 2/3 clinical study evaluating the efficacy and safety of THRV-1268, our best-in-class SGK1 inhibitor, in participants with Long QT Syndrome Type 2 (LQTS2). Wave II is now open for enrollment.
Wave III: Pivotal Phase 3 clinical study evaluating the efficacy and safety of THRV-1268, our best-in-class SGK1 inhibitor, in participants with Long QT Syndrome Type 3 (LQTS3). Enrollment is targeted to begin in 2027.
myQTwave Study:
myQTwave is a non-interventional, observational study designed to better understand the symptoms, lived experiences, and daily challenges of individuals living with Long QT Syndrome.
The study is currently enrolling adults living in the United States with LQTS Types 2 and 3. This initial phase of enrollment is capacity-limited; if the cohort is full, a waitlist is available, and additional participants will be incorporated as subsequent enrollment phases open.
Pre-Clinical Highlights:
In vitro evidence of QT-shortening: Our investigational SGK1 inhibitors reduced APD90 (Action Potential Duration at 90% repolarization, a surrogate for QT interval) in cardiomyocytes derived from patient iPSCs across all major LQTS subtypes: Type 1, 2, and 3.
Repeated positive effects observed in models of acquired (drug-induced) long QT.
Our Progress: Congenital Long QT Syndrome & Acquired (Drug-Induced) Long QT
Our work in Long QT Syndrome began with LQT-1213 and continues with our next-generation compound, THRV-1268, which incorporates learnings from early clinical success.
-
Congenital Long QT Syndrome is a rare genetic heart rhythm condition that lengthens the time for the heart to restore its electrical charge between beats. The QT interval is measured between the Q and T waves on an electrocardiogram (ECG or EKG). Prolongation of the QT interval increases an individual’s risk of developing an unexpected and life-threatening arrhythmia called “torsades de pointes”. People with Long QT Syndrome are at greatest risk to experience serious arrhythmias in response to exercise and stress.
-
A prolonged QT interval can occur in response to acute or chronic use of certain prescription medications. This is known as Acquired or Drug-Induced Long QT. These medications are prescribed when their benefits outweigh the risks of QT prolongation, especially when limited treatment options are available. The risk of QT prolongation with certain medications increases with underlying risk factors, including congenital Long QT Syndrome and/or abnormalities in bodily electrolytes (such as low potassium or low magnesium). These situations must be carefully assessed and managed by a healthcare provider.
Pre-Clinical Highlights:
Heart Failure:
Our investigational SGK1 inhibitors demonstrated therapeutic and protective effects in models of both HFpEF and HFrEF. Data presented at the 2025 American Heart Association (AHA) Scientific Sessions.
THRV-1268, our best-in-class SGK1 inhibitor, was shown to improve adverse cardiac remodeling better than Jardiance (empagliflozin), alone and in combination. Data presented at the 2025 American Heart Association (AHA) Scientific Sessions.
Atrial Fibrillation:
Our lead investigational SGK1 inhibitor, THRV-1268, demonstrated preventive effects in a high-fat diet-induced atrial fibrillation model. Data presented at the 2023 American Heart Association (AHA) Scientific Sessions.
Clinical Highlights:
Aspire Clinical Studies:
Phase 1 clinical study completed
Phase 2a proof-of-concept studies in a segmented population of heart failure with reduced ejection fraction (HFrEF). Enrollment is expected to begin in 2026.
Our Progress: Heart Failure & Atrial Fibrillation
-
Atrial fibrillation is a type of arrhythmia (irregular heart rhythm) which may present as a rapid, slow, or abnormal heart rate. These arrhythmias reduce the ability of the heart to efficiently to pump blood and may lead to blood clots localized in the heart which can dislodge and increase the risk of stroke, heart failure and other heart-related complications.
-
Heart failure is a chronic condition in which the heart's ability to pump blood effectively is impaired, either due to reduced systolic, pumping function (HF with reduced ejection fraction; HFrEF) or with normal systolic function but impaired diastolic filling between beats (HF with preserved ejection fraction; HFpEF). This diminished cardiac performance leads to inadequate blood flow to meet the body's needs, causing symptoms such as fatigue, shortness of breath, reduced ability to exercise, and fluid retention. The most common risks associated with the development of heart failure include coronary artery disease, heart attack (myocardial infarction), high blood pressure, obesity, smoking, excessive alcohol, physical inactivity, family history and genetic disorders.